
|
In order to provide our customers with excellent service, TIM Tuttlinger Instrumenten Manufaktur was founded in 1976 and renamed SUBAN Instruments Germany GmbH in 2021. The member company of the SUBAN Group is active in the distribution of surgical instruments in accordance with Article 14 of Regulation (EU) 2017/745 of SUBAN's own products and in the distribution of surgical components on behalf of and under the responsibility of the respective customers, and is a reliable partner and system service provider, located directly in the heart of the world center of medical technology in Tuttlingen. Its customer base includes world-renowned German and American partners.
SUBAN Instruments Germany GmbH has its own DIN EN ISO 13485 certification and is registered as a contract manufacturer with the FDA, which means it is approved for the US market and has been operating there for a long time. |
 |
 |
 |
 |
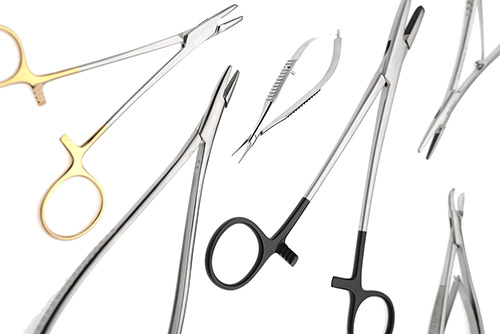
The products of the SUBAN group of companies are marked with chemical and laser technology. The Company has state-of-the-art laser marking machines, which are used to perform data matrix laser engraving on the instruments in accordance with the requirements of the UDI system and the expected MDR, in accordance with the GS1 standard. According to the regulations, the instruments are corrosion-resistant, wear-resistant and the markings can be read back with the recommended commercially available reading device during the entire life cycle of the product. |
FDA:8010665 CE: MDD 93/42 EEC according 14th of June, 1993 direkcíve. EN ISO 13485:2016 SUBAN Instruments Hungary Co. FDA:9680519 CE: MDD 93/42 EEC according 14th of June, 1993 directive. EN ISO 13485:2016 EN ISO 14001:2015 MSZ ISO 45001:2018 Russian registration:2015/2691 |
  |